
| Tweet |

Custom Search
|
|

|
||
 TM 9-8000
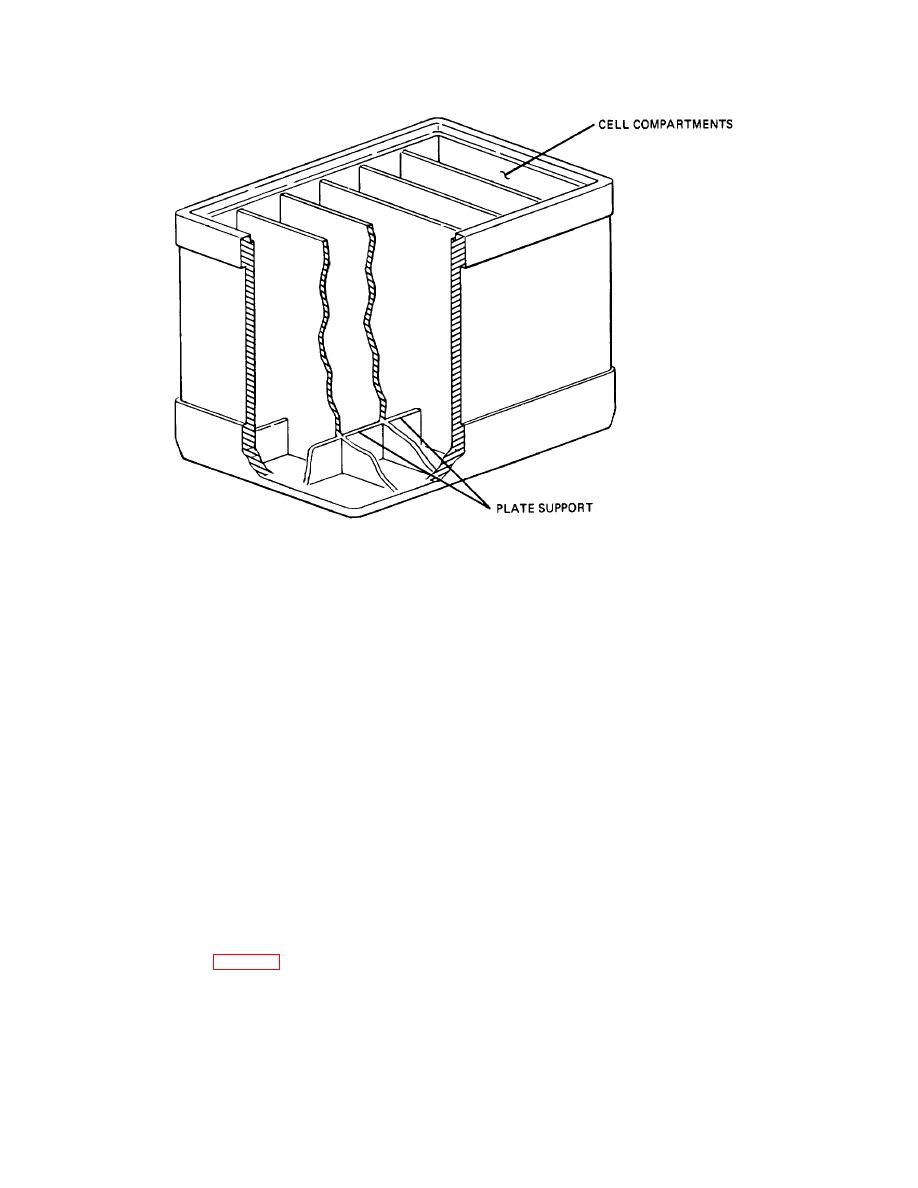
Figure 12-5. Battery Container Construction.
g. Cover. After all of the elements have been fitted
compartments for the individual cells. One element and
enough electrolyte to cover the plates are inserted into
into the case, they are connected together in series by
each cell compartment.
burning lead cell connectors across the terminals. The
battery top then is sealed with a hard rubber cover that
(2) Stiff ridges, or ribs, molded in the bottom of
provides openings for the two battery posts and a vent
the container form a support for the plates and a
plug for each cell. The vent plugs allow gas to escape
sediment recess for the flakes of active material that
and prevent the electrolyte from splashing outside the
drop off the plates during the life of the battery. The
battery. The battery is filled through the vent plug
sediment is thus kept clear of the plates so it will not
openings.
cause a short circuit across them.
Section II. PRINCIPLES OF OPERATION
electrolyte becomes weaker during discharge, because
12-3. General. When a cell is fully charged, the
the water increases and the sulfuric acid decreases. As
negative plate is spongy lead, the positive plate is lead
the discharge continues, the negative and the positive
peroxide, and the electrolyte contains a maximum
plates finally contain considerable lead sulfate and the
amount of sulfuric acid. Both the negative and positive
electrolyte turns to almost pure water. At this point the
plates are very porous and are acted upon readily by the
battery will stop providing current flow.
acid. A cell in this condition can produce electrical
energy through reaction of the chemicals.
12-5. Charge.
12-4. Discharge. If the terminals of the battery are
a. Chemical Action. To charge the cell, an external
connected to a closed circuit, the cell discharges to
source of direct current must be connected to the battery
supply electric current (fig. 12-6). The chemical
terminals. The chemical reaction is reversed then and
process that occurs during discharge changes both the
returns the positive
lead of the negative plate and the lead peroxide of the
positive plate to
lead sulfate and the sulfuric acid to water. Thus, the
TA233538
12-4
|
||
 |
||