
| Tweet |

Custom Search
|
|

|
||
 TM 9-8000
during its growth, there is no measurable pressure
further compressed and are heated to high
created by heat.
temperatures. Under certain conditions, the extreme
heating of the unburned part of the mixture may cause it
(2) Hatching Out. As the nucleus enlarges, it
to Ignite spontaneously and explode.
This rapid,
uncontrolled burning in the final stage of combustion is
develops into the hatching out stage. The nucleus is torn
called detonation. It is caused by the rapidly burning
apart so that it sends fingers of flame into the mixture in
flame front compressing the unburned part of the mixture
the combustion chamber. This causes enough heat to
to the point of self-ignition. This secondary wave front
give just a slight rise in the temperature and pressure In
collides with the normal wave front, making an audible
the entire air-fuel mixture. Consequently, a lag still exists
knock or ping. It is an uncontrolled explosion, causing
in the attempt to raise pressure in the entire cylinder.
the unconfined gases in the combustion chamber to rap
(3) Propagation. It is during the third, or
against the cylinder head walls. Detonation may harm
an engine or hinder its performance in several ways. In
propagation, stage that effective burning occurs. The
extreme cases, pistons have been shattered, rings
flame now burns in a front that sweeps across the
broken, or heads cracked. Detonation also may cause
combustion chamber, burning rapidly and causing great
overheating, excessive bearing wear, loss of power, and
heat with an accompanying rise in pressure. This
high fuel consumption.
pressure causes the piston to move downward. The
burning during normal combustion is progressive. It
d.
Octane Rating.
increases gradually during the first two stages, but during
the third stage, the flame is extremely strong as it
(1) The ability of a fuel to resist detonation is
sweeps through the combustion chamber.
measured by its octane rating. The octane rating of a
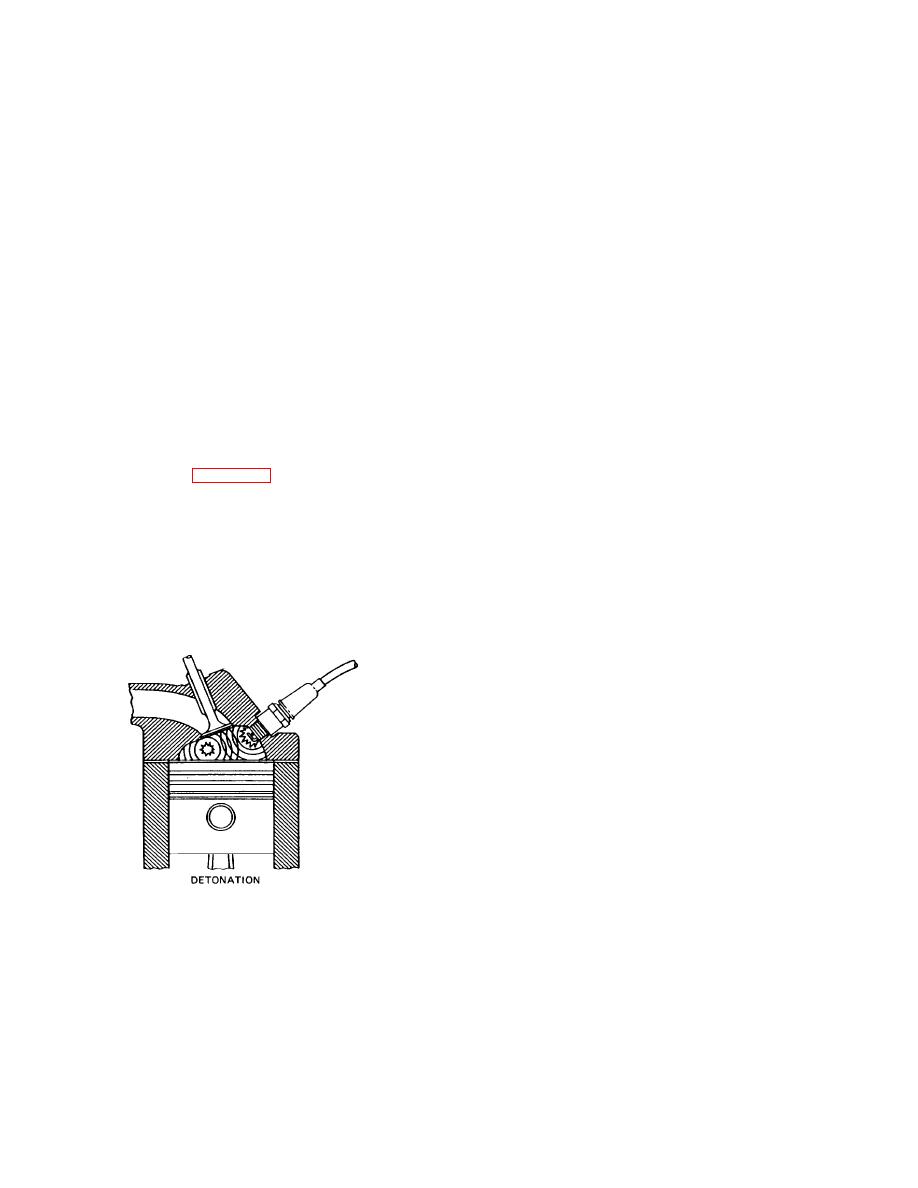
c. Detonation (Fig. 4-77). If detonation takes
fuel is determined by matching it against mixtures of
normal heptane and iso-octane in a test engine under
place, it will happen during the third stage of combustion.
specified test conditions until a pure mixture of
The first two stages are normal, but In the propagation
hydrocarbons is found that gives the same degree of
stage, the flame sweeps from the area around the spark
knocking in the engine as the gasoline being tested. The
plug toward the walls of the combustion chamber. Parts
octane number of the gasoline then is specified as the
of the chamber that the flame has passed contain inert
percent of the isooctane In the matching iso-octane,
gases, but the section not yet touched by the flame
normal heptane mixture. For example, a gasoline rating
contains highly compressed, heated combustible gases.
of 75 octane is equivalent in its knocking characteristics
As the flame races through the combustion chamber, the
to a mixture of 75 percent iso-octane and 25 percent
unburned gases ahead of it are
normal heptane. Thus, by definition, normal heptane has
an octane rating of 0 and isooctane has an octane of
100.
(2) The tendency of a fuel to detonate varies in
different engines and in the same engines under different
operating conditions. The octane number has nothing to
do with starting qualities, potential energy, volatility, or
other major characteristics. Engines are designed to
operate within a certain octane range. Performance is
improved with the use of higher octane fuels within that
operational range. Engine performance will not be
improved if a gasoline with an octane rating higher than
the operational range is provided.
(3) Tetraethyl lead is the most popular of the
compounds added to gasoline to raise its octane rating.
Figure 4-77. Detonation.
The introduction of catalytic con-
TA233437
4-54
|
||
 |
||