
| Tweet |

Custom Search
|
|

|
||
 TM 9-8000
at -144F (-97.8C) and ethyl alcohol, which freezes at -
methyl alcohol, and ethyl alcohol. Ethyl and methyl
174F (-114.3C).
alcohol provide adequate protection as an antifreeze
when used in sufficient quantities. The main objection to
these liquids, however, is that they evaporate below the
b. Corrosion Resistance. The cooling system must
operating temperature of modern automotive engines,
be free of rust and scale in order to maintain its
making them impractical. Glycerin offers the same
efficiency. The use of inhibitors or rust preventatives will
degree of protection as alcohol, but does not evaporate
reduce or prevent corrosion and the formation of scale.
in use because of its high boiling point. Ethylene glycol
Inhibitors are not cleaners and therefore will not remove
(antifreeze compound) has an extremely high boiling
rust and scale that have already accumulated. Most
point, does not evaporate in use, is noncorrosive, has no
commercially available antifreeze solutions contain
odor, and gives complete protection against freezing in
inhibitors. If water alone is used as a coolant, an
normal use. Ethylene glycol gives a maximum protection
inhibitor should be added.
against freezing to - 65F ( - 53.8C) when it is mixed to
a solution of 60 percent with 40 percent water.
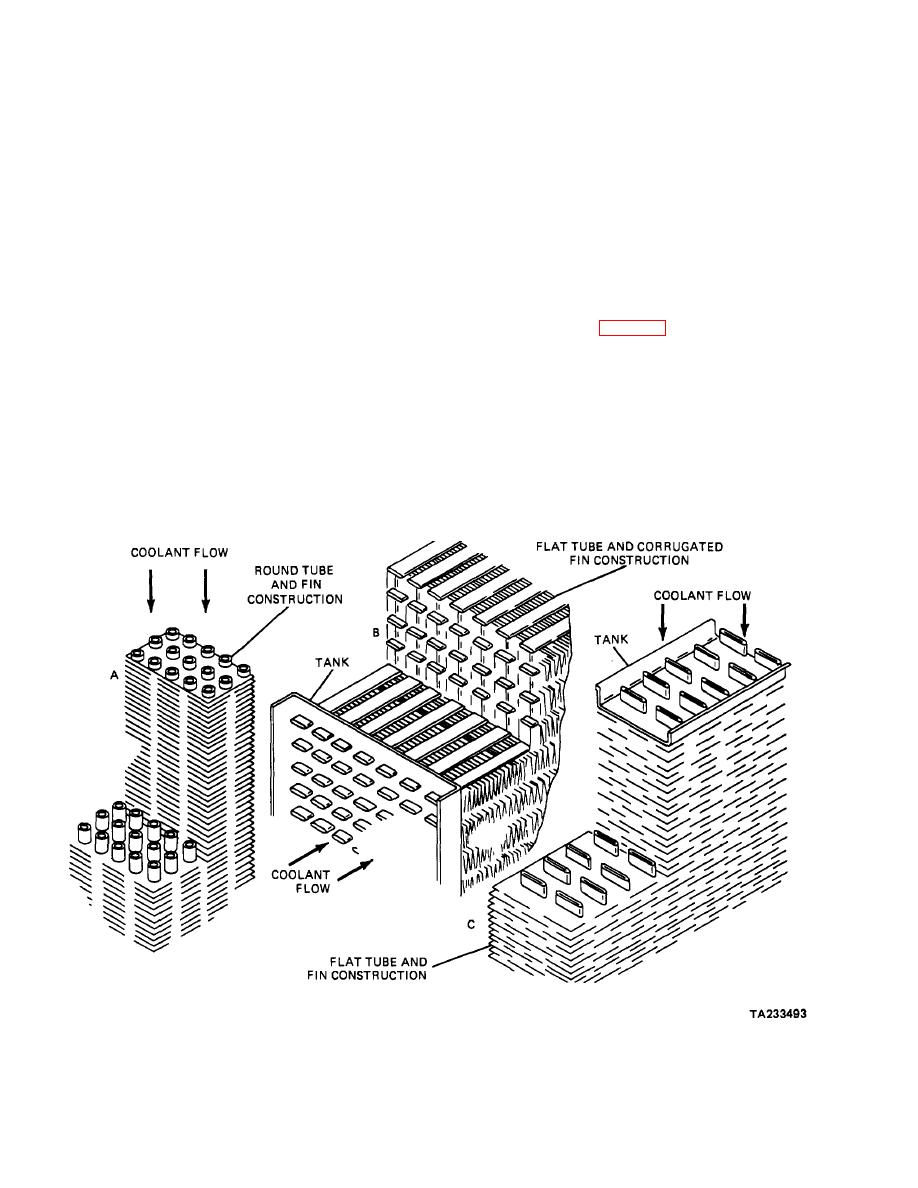
vehicles using liquid cooling systems consist of two tanks
If the proportions of ethylene glycol are raised in the
with a heat exchanging core between them. The upper
solution, it will result in a higher freezing point for the
tank contains an outside pipe called an inlet. The filler
solution, consequently giving less protection. If a 100-
neck generally is placed on the top of the upper tank;
percent solution of ethylene glycol were used, its freezing
attached to this filler neck is an outlet to the overflow
point would not be much below that of water. Other
pipe. The lower tank also contains an outside pipe that
antifreeze solutions, however, do not show this increase
serves as the radiator's outlet. Operation of the radiator
of freezing point with increasing concentration. Two
is as follows.
good examples are methyl alcohol, which freezes
a.
The upper tank collects incoming coolant
Figure 9-2. Engine Radiator Construction
9-3
|
||
 |
||