
| Tweet |

Custom Search
|
|

|
||
 TM 9-8000
CHAPTER 20
HYDRAULIC PRINCIPLES
Section I. PRINCIPLES
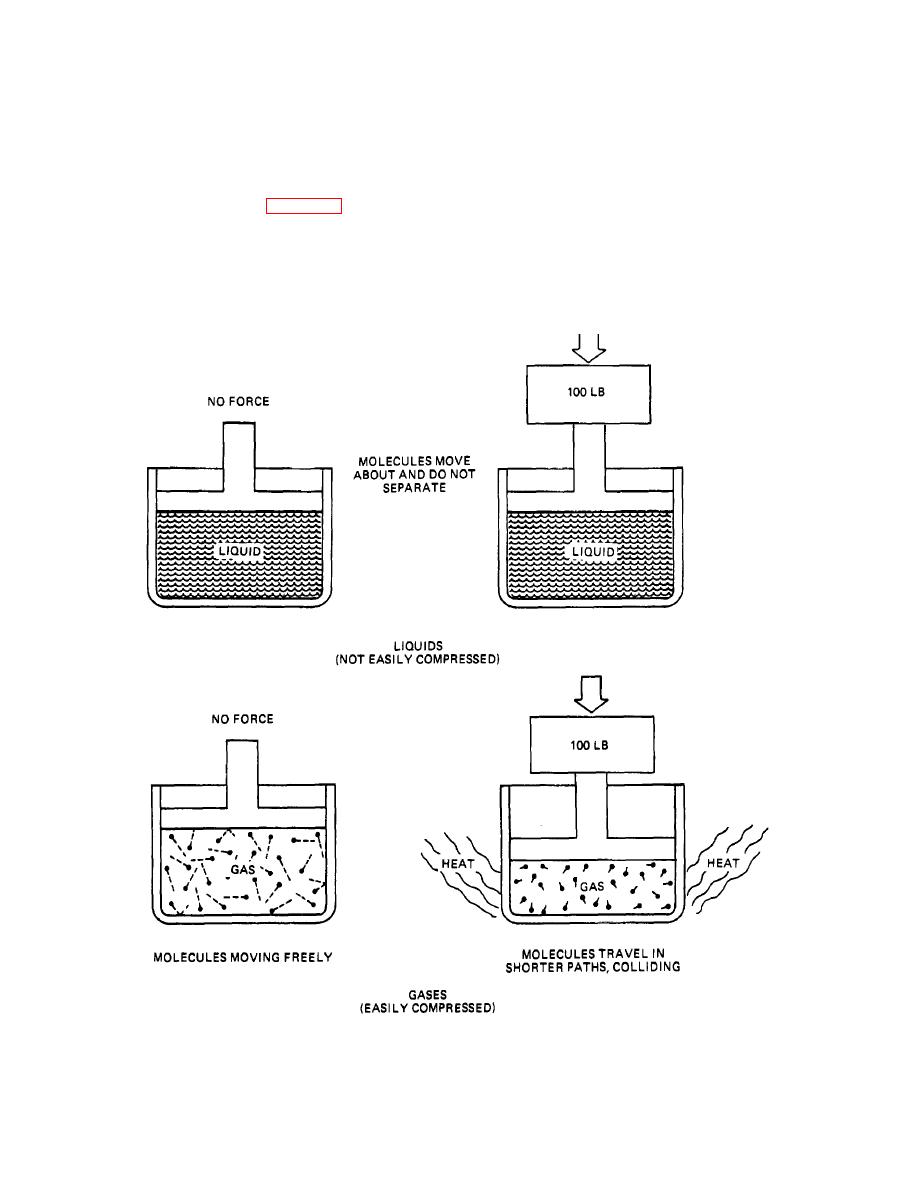
separately, their molecules act differently. Liquids have
molecules that travel very short distances with almost no
factor that influences the compressibility of liquids and
space between them. Attempting to compress the liquids
gases Is their molecular bonding. Liquids have molecules
yields almost no change In the space between
moving freely around them, but not separating. Gases
molecules. Therefore, the volume also remains constant.
have molecules that are more active and tend to
This is the major reason why liquids are not compressed
separate readily. Because both liquids and gases are
compressed
Figure 20-1. Compressibility of Gases and Liquids.
20-1
|
||
 |
||